
Read this before leaf tissue testing for micronutrients
26th February 2019Views from: Jim Orson, BCPC Executive Board
The great Gordon Banks is dead. I regularly sat behind the goal at Leicester City’s Filbert Street ground in the very early 1960’s and witnessed his sublime skills. The fulsome tributes reflect that he was blessed with a true richness of talent which, through hard work, he was able to express on the football pitch.
There are often other attributes people need in order that they can exploit their talents to the full. Like Gordon Banks, it may simply be hard work but for others, it may also be identifying the right opportunity, if and when it comes along. I was thinking about this the other day whilst preparing for a talk. It is clear that the UK is blessed with a climate and mineral rich soils that can support high yields but we were only able to express this potential once effective crop protection evolved. Now, we regularly feature in the world’s top three or four countries for wheat yield/ha. This success has come at the cost of criticism or indeed hostility towards the methods we have adopted. Despite this, hard scientific evidence clearly shows that if we are to increase food production at a rate to reflect our own growing population then high yield farming is essential to make space for nature.
As I said earlier, there are always those wishing to criticise conventional farming. All kinds of lurid tales are thrown at the industry. The common accusation on the website www.wewantrealfood.com is that conventional farming is stripping our soils of the essential minerals for growth and for the health of the consumer. The evidence from Rothamsted for wheat in a paper written over ten years ago is that the soil content of micronutrients had not changed over 160 years of the Broadbalk experiment but higher yields have diluted the content of some minerals in the harvested grain.
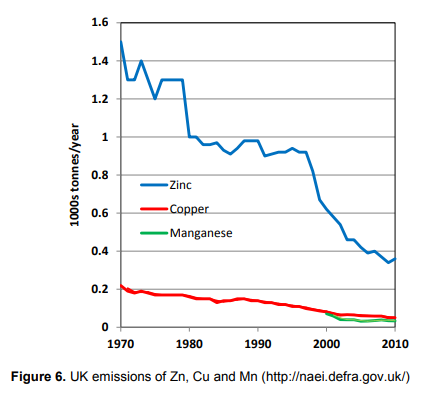 Over the previous 100 years or so, this lack of a fall in the soil content of some micronutrients may have been due to partly their industrial emissions. These emissions are now rapidly falling, which suggests that there is perhaps a need to monitor soil content in the medium to long term. The graph, based on Defra data, is taken from AHDB Project Report 518. It reminds me of the reduction in sulphur emissions we saw two or three decades ago. This Rothamsted led project report published five years ago concluded that levels of copper and zinc in the soil may have fallen slightly over the previous 25 years but to such a small extent that it had no practical significance. So levels in the soil may be changing but very, very slowly.
Over the previous 100 years or so, this lack of a fall in the soil content of some micronutrients may have been due to partly their industrial emissions. These emissions are now rapidly falling, which suggests that there is perhaps a need to monitor soil content in the medium to long term. The graph, based on Defra data, is taken from AHDB Project Report 518. It reminds me of the reduction in sulphur emissions we saw two or three decades ago. This Rothamsted led project report published five years ago concluded that levels of copper and zinc in the soil may have fallen slightly over the previous 25 years but to such a small extent that it had no practical significance. So levels in the soil may be changing but very, very slowly.
The same project investigated the value to winter wheat of two foliar spray applications of copper, zinc or manganese on sites where deficiencies are most likely to occur i.e. organic and/or sandy soils with a high pH where organic amendments containing these micronutrients are not applied. There were five field sites a year in the three year project. Despite the sites being located on soils most likely to have a deficiency, there was only one statistically significant yield increase with the application of copper and one with zinc in the 15 trials.
There has subsequently been a huge argument over these results. The basis of the debate is whether or not some slight yield ‘increases’ were real or were just a reflection of the natural variation within an individual site. The problem is that micronutrient sprays are reasonably cheap and the level of yield response required for an economic treatment can be below what can be assessed as statistically significant.
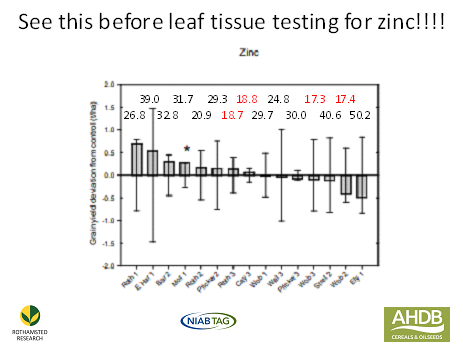 I have been looking through the data from these 15 trials and have come to the conclusion that the two statistically significant yield increases were the only true responses to the micronutrient sprays. My reason for saying this is that the two sites had levels of zinc or copper in the soil and grain at deficiency levels. However, leaf tissue analysis of samples collected in the spring, at the time recommended by the commercial partners in the project, was pretty hopeless in predicting deficiency. Just look at the histogram for the ‘yield responses’ to zinc at each of the 15 sites. Above each ‘yield response’ column are the results of the leaf tissue analysis in milligrams/kilogram and the numbers in red denote ‘deficiency’. Enough said, except to point out that the site which gave the statistically significant response (starred) had satisfactory levels in the leaf tissue at the time of sampling but soil and grain analysis showed that this site would have been likely to respond to a spray treatment.
I have been looking through the data from these 15 trials and have come to the conclusion that the two statistically significant yield increases were the only true responses to the micronutrient sprays. My reason for saying this is that the two sites had levels of zinc or copper in the soil and grain at deficiency levels. However, leaf tissue analysis of samples collected in the spring, at the time recommended by the commercial partners in the project, was pretty hopeless in predicting deficiency. Just look at the histogram for the ‘yield responses’ to zinc at each of the 15 sites. Above each ‘yield response’ column are the results of the leaf tissue analysis in milligrams/kilogram and the numbers in red denote ‘deficiency’. Enough said, except to point out that the site which gave the statistically significant response (starred) had satisfactory levels in the leaf tissue at the time of sampling but soil and grain analysis showed that this site would have been likely to respond to a spray treatment.
Overall, leaf tissue analysis suggested a deficiency of either zinc or copper in seven of the fifteen sites; four for zinc and three for copper but all but one of these sites did not respond to treatment. The high and statistically significant response to copper on one site was predicted by leaf tissue analysis but there were very similar levels in the leaf tissue at two other sites that did not respond at all. Grain and soil analysis show that this was a deficient site.
When you really think about it, the unacceptable ability of leaf tissue analysis to identify responsive sites to micronutrient sprays is not surprising. A leaf tissue test in the spring is a mere snapshot of the micronutrient content in a constantly changing crop whilst soil analysis and grain analysis reflect copper and zinc availability for the whole season.
In my opinion, this AHDB project clearly shows that only soil and grain analyses are good identifiers of sites that are most likely to respond to a micronutrient treatment. On the other hand, leaf tissue analysis is far more likely to miss responsive sites to sprays and result in a lot of money wasted on false recommendations to spray. Make sure that the soil or grain analysis is done using the correct laboratory technique and also ensure that the correct standards are used to indicate deficiency. I have to say that my conclusions on the (lack of) value of leaf tissue testing are not new. It says as much on page 26 of section 4 of RB 209.
An exception when using soil analysis as a predictor of deficiency is manganese. It only indicates the soluble manganese at the time of sampling, not what will happen later. Luckily, even in the presence of mild deficiency symptoms, experiments have suggested that there is no yield response to a manganese spray but the crop can look awful. In the short term, a mild deficiency may result in a floppy and more disease ridden crop canopy that may be more susceptible to damage from pesticides.
I have also learnt from the AHDB project that, in contrast to manganese, yield responses to the application of zinc and copper can occur in the absence of deficiency symptoms. Hence, particularly in view of falling levels of atmospheric deposition, it is worth monitoring, say once in every four or five years, levels in grain or soil in situations where deficiencies are most likely to occur. It may be that grain analysis is the preferred option for long term monitoring, provided of course that micronutrient sprays were not applied to the sampled crop. This is because grain analysis has a better chance than soil sampling of achieving a truly representative sample of a field.
If you have any questions about this blog I can be contacted on jim.orson@niab.com.

